2.4 Dilutions of Standard Solutions
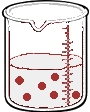
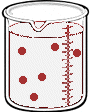
Imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt (a certain mass or a certain number of moles) dissolved in a certain volume of solution. Next we'll dilute this solution - we do that by adding more water, not more salt:
Before Dilution |
After Dilution |
|||||
 |
 |
|||||
Solution1 |
Solution2 |
|||||
|
|
|||||
rearrange the equations to find moles: |
||||||
| moles1 = M1× litre1 | moles2= M2 × litre2 | |||||
What stayed the same and what changed between the two solutions?
By adding more water, we changed the volume of the solution. Doing so also changed it's concentration. But the number of moles of solute did not change. So,
moles1 = moles2
Therefore
M1 × litre1 = M2 × litre2
Which we will usually express as:
M1V1= M2V2 where M1 and M2 are the concentrations of the original and diluted solutions and V1 and V2 are the volumes of the two solutions |
Preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand the above relationship, the calculations are easy to do.
Example:
What volume of concentrated sulfuric acid, 18.0 M, is required to prepare 5.00 L of 0.150 M solution by dilution with water?
Solution:
In a dilution question there are 4 variables - M1, V1, M2 and V2. You will know three of these values and have to calculate the fourth. Organizing your information is the key to correctly answering dilution questions. The most common mistake students make is in incorrectly pairing up concentrations and volumes. Take time to make sure you do not make this mistake.
M1 = 18.0 M M2= 0.150 M V1 = ? V2 = 5.00 L Set up the formula, and rearrange to solve for the unknown, V1:
M1V1 M2V2 18.0 × V1 0.150 × 5.00 V1
18.0V1 0.0417 L 41.7 mL Standard Solutions
It is often necessary to have a solution whose concentration is very precisely know. Solutions containing a precise mass of solute in a precise volume of solution are called standard solutions.
|
|
|
Example:
Describe how you would prepare 500.0 mL of a 0.100 M standard solution of KNO3
Solution:
We must first determine the mass of KNO3 that will be needed to prepare 500.0 mL (0.500 L) of a 0.100 M solution. The molar mass of KNO3 is 101.1 g·mol-1:
g |
= |
101.1 g
|
× |
0.100
|
× |
0.500 |
= |
5.05 g |
To prepare the standard solution we will carefully measure out 5.05 g of KNO3 and dissolve it in some water (perhaps 200 mL of water). We pour this solution into a 500 mL volumetric flask and add just enough water reach the calibration mark on the flask.
Complete the practice questions before moving on to the next page.
