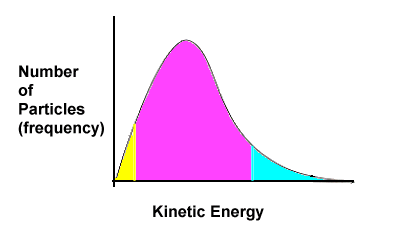
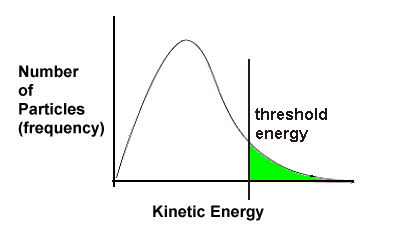
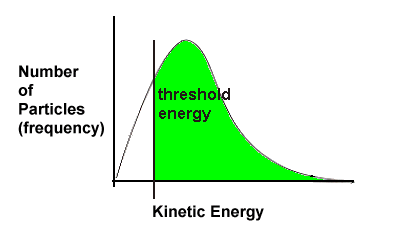
2.3 Threshold Energy
Let's return to one of the key points of the collision theory - reacting particles must collide with sufficient force or energy.
Consider two cars about to become involved in an accident. If both cars are moving very slowly, say 2 km/hr when they collide, not much is going to happen. Perhaps the drivers don't even realize they've hit one another. But if the cars are both travelling at 40 km/hr (the greater speed indicates the cars have more energy), then the collision is going to be effective and damage will be done. In our scenario there is a certain speed at which damage will be done to the cars. In chemistry, we call this minimum amount of energy required for particles to collide successfully activation energy. |
|