| 1. | Phosgene, COCl2, one of the poison gases used during World War I, is formed from chlorine and carbon monoxide. The mechanism is thought to proceed by:
a. Write the overall reaction equation. b. Identify any reaction intermediates. c. Identify any catalysts. |
|||||||||||||||||||||||||||||||
a. The overall reaction:
|
||||||||||||||||||||||||||||||||
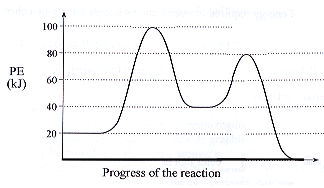
| 2. | We have typically been simplifying our potential energy curves somewhat; for multistep reactions, potential energy curves are more accurately shown with multiple peaks. Each peak represents the activated complex for an individual step. Consider the PE curve for a two-step reaction:
|
 |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||