4.4 Factors Influencing Reaction Rate: Catalysts
When solid potassium chlorate is heated, potassium chloride and oxygen are produced. The reaction is not particularly fast.
2 KClO3 (s) → 2 KCl (s) + 3 O2(g)
This reaction can take place much more rapidly, and at lower temperatures, if solid manganese dioxide (MnO2) is added. After the reaction is complete, no more potassium chlorate remains, but all of the manganese dioxide remains.
Catalysts are very specific regarding which reactions they work with - the same catalyst will not necessarily work with many other (or any other) reactions. Finding a catalyst for a specific equation is one of the important jobs of an industrial chemist.
Catalysts most likely work by helping to promote a proper orientation between reacting particles. In doing so, it provides an alternate reaction pathway with a lower activation energy.
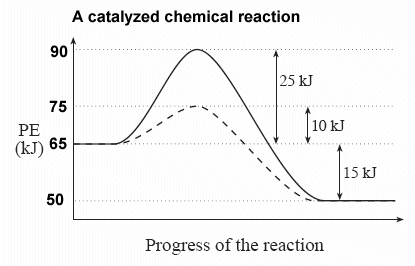
In the potential energy diagram shown here, the regular, uncatalyzed pathway is shown as a solid line, and the catalyzed pathway is shown as the dotted line:
ΔH for the both the catalyzed and uncatalyzed reaction is -15 kJ.
Activation energy, Ea, does have different values for the catalyzed and uncatalyzed reactions.
Ea for the uncatalyzed reaction = +25 kJ
Ea for the catalyzed reaction = +10 kJ
Because more particles will possess the new 10 kJ energy minimum for a successful collision, the rate of the reaction will increase.
It is important to note that the original pathway is still present, and reacting particles will still follow that route.
Think of a catalyst as a new shortcut in your walk to school, or work, or whatever. The longer road still exists and can be used, but the shortcut makes for a faster trip.
Here's another example:
2 N2O → 2 N2 + O2
Ea for the uncatalyzed reaction is 250 kJ, but when a gold metal catalyst is used, Ea is lowered to 120 kJ.
The N2O is chemically adsorbed on the metal surface. A bond is formed between the O of the N2O and an Au atom. This weakens the bond joining the O to the N, thus making it easier for the molecule to break apart.
A Catalyzed Reaction
The Breakdown of Ozone
by Freon
The movie may take a
few minutes to download
Source:
Petrucci, Ralph H., William S. Harwood, Geoffrey Herring. General Chemistry: Principles and Modern Applications, Eight Edition. Prentice Hall.
A Catalyzed Reaction -
The Breakdown of Ozone
NO Catalyst
The movie may take a
few minutes to download
Source:
Petrucci, Ralph H., William S. Harwood, Geoffrey Herring. General Chemistry: Principles and Modern Applications, Eight Edition. Prentice Hall.
In addition to kinetic energy there is another basic form of energy - potential energy. Potential energy is energy of position; we often refer to it as stored energy.
Kinetic Energy
Energy of motion.A rock rolling down a hill has kinetic energy.
Atoms and molecules have kinetic energy as they are always in motion
(click here for more information).Potential Energy
Stored energy.A rock sitting on top of the hill has stored energy.
Inhibitors
Some substances, known as inhibitors, slow down chemical reactions. These work by tying the reactants up in "side reactions".
Complete the Practice Questions and check your answers. Then complete the assignment.