| 1. | Answer the following questions based on the potential energy diagram shown here:
|
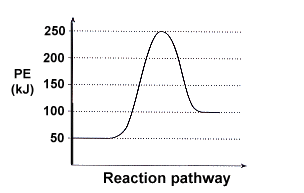 |
| 2. | Sketch a potential energy curve that is represented by the following values of ΔH and Ea. You may make up appropriate values for the y-axis (potential energy).
Is this an endothermic or exothermic reaction? |
|
| 3. | In the next unit we will be discussing reactions that are reversible, and can go in either the forward or reverse directions. For example, hydrogen gas and oxygen gas react to form water, but water can also be broken down into hydrogen and oxygen gas. We typically write a reaction that can be reversed this way, using the double arrow symbol ( or ↔): 2 H2 + O2 ↔ 2 H2O
2 H2 + O2 ↔ 2 H2O + 285 kJ
2 H2O + 285 kJ ↔ 2 H2 + O2 Consider a general reversible reaction such as: A + B ↔ C + D
|
|
| Given the following potential energy diagram for this reaction, determine ΔH and Ea for both the forward and reverse directions. Is the forward reaction endothermic or exothermic? | 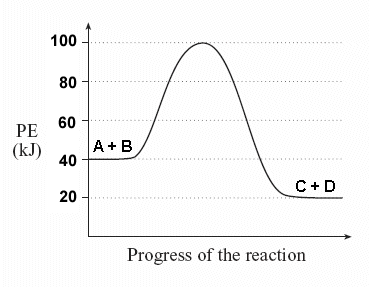 |
|
| 4. | Sketch a potential energy diagram for a general reaction A + B ↔ C + D Given that ΔHreverse = -10 kJ and Ea forward = +40 kJ |
|
Chemistry 30
Thermodynamics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Kinetics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Equilibrium: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Solutions: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Acids & Bases: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Redox Reactions: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources