Print out the lab : Word Format | RTF Format | PDF Format |
Overview
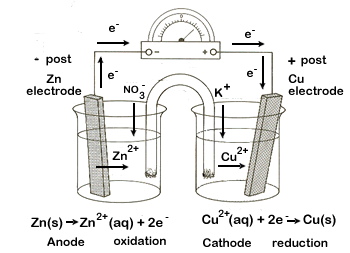
In an electrochemical cell, chemical energy is converted into electrical energy. This is accomplished by using a spontaneous chemical reaction to generate an electric current, which we can simply define here as electrons traveling though a wire.
To create the electrochemical cell two half-reactions will be set up in different containers. In one, an oxidation reaction will be used to generate a source of electrons. These free electrons will travel, through an external circuit, to the second container and will cause the reduction reaction to occur. The final requirement for our complete electrochemical cell will be a salt bridge that will permit ions to flow between the two half-cells, thus maintaining electrically neutral solutions.
Purpose
- To build several electrochemical cells.
Safety
- Follow normal lab safety guidelines. There are no specific safety hazards for this lab.
Equipment and Materials
Equipment
- 250-mL beakers, 2
- glass U-tube for the salt bridge
- cotton plugs for the salt bridge
- copper wires, 2, insulated, with alligator clips
- steel wool to clean electrodes
- DC voltmeter
- metal electrodes: copper, zinc, lead
Solutions
- 0.5M Cu(NO3)2
- 0.5M Zn(NO3)2
- 0.5M Pb(NO3)2
- 0.5M KNO3- for the salt bridge
Procedure
- Each half-cell will be created by placing a metal electrode in an electrolytic solution containing the same metal's ions. For example the copper electrode will be placed in a copper(II) nitrate solution.
For each electrochemical cell you create you will require two half-cells. Set these cells beside each other - they will be connected by the U-tube.
- Fill a beaker about two-thirds full of the electrolytic solution. Clean the electrode using the steel wool, then place the electrode in its appropriate solution.
- Clip one end of each copper wire to the two electrodes using the alligator clips.
- Fill the U-tube with KNO3 and stopper both ends with the cotton plugs. Turn the U-tube upside down and place one end in each half-cell.
- Touch the other end of the copper wires to the voltmeter terminals. If the indicator on the voltmeter deflects in the wrong direction, switch the wires on the terminals. Read the highest voltage reading obtained - you'll need to do this quickly after connecting the wires to the voltmeter.
- Repeat the experiment for other combinations of half-cells.
Results
Copy a data table similar to the one shown below into your lab notebook and use it to record your results.
Half-cells |
Voltage |
|
Cu|Cu2+ |
Zn|Zn2+ |
|
Cu|Cu2+ |
Pb|Pb2+ |
|
Zn|Zn2+ |
Pb|Pb2+ |
|
Questions and Conclusions
- For each electrochemical cell you created:
- Write out the two half-reactions for each electrochemical cell you created.
- Identify each half-reaction as oxidation or reduction.
- Identify each half-reaction as the anode and cathode.
- Indicate the direction of the flow of electrons
- Using a Table of Standard Reduction Potentials, calculate the theoretical voltage for each cell.
- Compare the voltages you obtained with the theoretical voltage for each cell. What are some reasons that would account for any differences?