2.2 Electrochemical Cells
The basic unit of all batteries is the electrochemical cell (also called a voltaic cell or galvanic cell). Electrochemical cells convert the energy of a spontaneous redox reaction into electricity. This will be accomplished as the electrons that are released from the oxidation half-reaction are passed to the reduction reaction which will absorb the electrons.
We will create an electrochemical cell based on the following redox reaction:
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
This reactions involves two half-reactions:
Zn → Zn +2 + 2 e- |
Cu2+ + 2e- → Cu |
oxidation |
reduction |
In order for electrical work to be done by this reaction, we need to have the electrons travel through an external circuit. If we simply placed a piece of zinc metal in a solution containing copper(II) ions, a reaction would occur but electricity would not be created.
We'll walk through the set-up of our electrochemical cell:
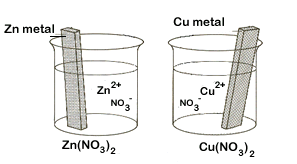
| 1. | Begin by getting 2 beakers into which we will place metal strips in electrolytic solutions (solutions that conduct electricity due to the presence of ions). In one place a strip of zinc metal in a Zn(NO3)2 solution. In the other place a strip of copper metal in a Cu(NO3)2 solution. | ||||||
Each beaker represents one of the two half cells. But because there is no way for electrons to move from one beaker to the other, our redox reaction cannot yet occur. |
 |
||||||
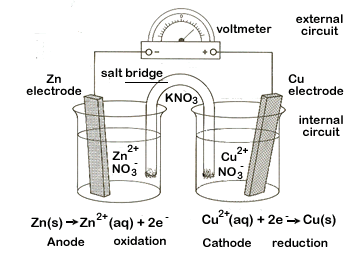
| 2. | We need to connect our two half-cells which we need to do in two ways. | ||||||
| First we will connect the two metal strips, our electrodes, with some wire. We'll also place a voltmeter here so we can detect the electric current once we are up and running. This will be our external circuit. | |||||||
Second we add a salt bridge. A salt bridge is a U-shaped tube that contains an electrolytic solution (we'll use KNO3). This electrolytic solution will allow ions to flow between the two beakers. This is our internal circuit.
|
 |
||||||
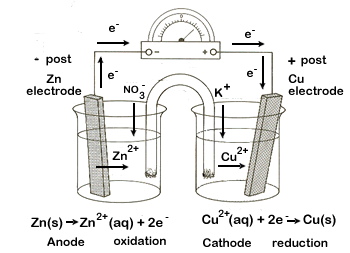
| 3. |  An Ox Anode = Oxidation |
The zinc half-cell undergoes oxidation. Here, the solid zinc electrode disintegrates, forming zinc ions and releasing electrons. By definition, the half-reaction that undergoes oxidation in an electrochemical cell is called the anode. The anode is the source of electrons, making it the negative post of the electrochemical cell. |
|||||
|
|||||||
| 4. | It is important to understand the roles of the external circuit and the salt bridge. | ||||||
| External circuit - this is where the electrical work is done as electrons travel from one half-cell to the other. The electrons are produced at the zinc anode, where oxidation occurs. The electrons then travel through the wire of the external circuit to the copper cathode. The electrons are then available for the copper ions (from the Cu(NO3)2 solution) and solid copper is produced. | |||||||
Internal circuit - At the anode, Zn2+ ions are being produced and go into solution. This causes a build-up of positive ions in this solution. If this electrical imbalance is not corrected the reaction cannot continue. The excess positive charge attracts the negative NO3- ions (anions) from the salt bridge, thereby keeping the solution electrically neutral.
|
 |
||||||
| At the cathode the opposite occurs. As positive Cu2+ ions are removed from solution, to form solid Cu, the solution becomes overly negative. This attracts the positive K+ cations from the salt bridge, keeping this side of the cell neutral. | |||||||
|
|||||||
Once we have the entire electrochemical cell assembled - the two half-cells (the electrodes in their electrolytic solutions), the internal circuit (the salt bridge and half-cells), and the external circuit (the wire connected the two electrodes) - the cell is complete and the redox reaction will occur.
It is important to note and remember that unless the electrons can pass from one electrode to the other the reaction will not proceed.
There's a lot to remember when setting up electrochemical cells and it will take some practice to remember all of the details. Before we do that, however, there are a few more items to cover.
