Print out the practice set Word RTF Print out the answers Word RTF
For questions 1 to 3, two half-cells are connected under standard conditions to make an electrochemical cell. Use the Table of Standard Reduction Potentials included with this assignment to obtain the half-reactions involved. For each:
a. write the equation for each half-reaction that will occur
b. label each half-reaction as oxidation or reduction
c. calculate the voltage of the electrochemical cell
d. the net overall balanced redox equation.
e. diagram the cell, clearly indicating the following
- the electrodes in appropriate electrolytic solutions
- label each electrode as anode or cathode
- label each electrode as positive post or negative post
- diagram the flow of electrons through the external circuit
- a salt bridge with appropriate electrolytic solution
- flow of ions from the salt bridge to the two half-cells
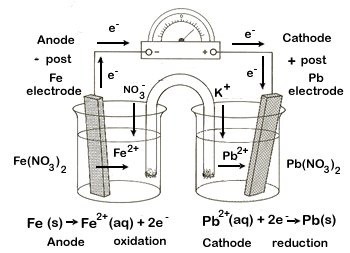
1. iron-iron(II) ion (Fe|Fe2+) and lead-lead(II) ion (Pb|Pb2+)
|
|
|
E°(V) |
|
cathode |
reduction |
Pb2+ + 2e- → Pb(s) |
-0.13 |
+ electrode |
anode |
oxidation |
Fe(s) → Fe2+ + 2e- |
+0.45 |
- electrode |
balanced net |
Fe(s) + Pb2+ → Pb(s) + Fe2+ |
+0.32 |
total |
|
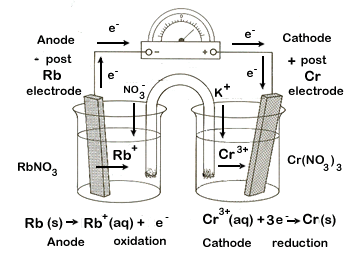
| 2. chromium-chromium(III) ion (Cr|Cr3+) and rubidium-rubidium ion (Rb|Rb+) | |||||
|
|
balance for e- |
|
E° (V) |
|
cathode |
reduction |
|
Cr3+ + 3e- → Cr(s) |
-0.74 |
+ electrode |
anode |
oxidation |
× 3 |
Rb(s) → Rb+ + e- |
+2.98 |
- electrode |
balanced net |
3 Rb(s) + Cr3+ → Cr(s) + 3Rb+ |
+2.24 |
total |
||
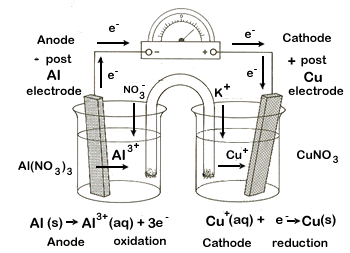
| 3. copper-copper(I) ion (Cu|Cu+) and aluminum-aluminum ion (Al|Al3+) | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
|
balance for e- |
|
|
E° (V) |
|
||||||||||||||||||||||||||||||||||||
cathode |
reduction |
× 3 |
Cu+ + e- → Cu(s) |
|
+0.52 |
+ electrode |
||||||||||||||||||||||||||||||||||||
anode |
oxidation |
|
Al(s) → Al3+ + 3 e- |
|
+1.66 |
- electrode |
||||||||||||||||||||||||||||||||||||
balanced net |
Al(s) + 3 Cu+ → 3Cu(s) + Al3+ |
|
+2.18 |
total |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Part a. | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Part b. | ||||||||||||||||||||||||||||||||||||||||||
The balanced equation is required to answer part b:
|
||||||||||||||||||||||||||||||||||||||||||