2.1 Heat Content - Enthalpy
Let's put a name to the branch of chemistry we have been studying–Thermodynamics. Thermodynamics is the study of energy changes during chemical reactions, and the influence of temperature on those changes.
Here's another important term — Enthalpy
Enthalpy is the heat content of a system, or the amount of energy within a substance, both kinetic and potential.
Every substance possesses both stored energy, due to the nature of the chemical (and nuclear) bonds holding the substance together, and kinetic energy which arises from to the constant motion of the particles. This total amount of energy is enthalpy.
It should be noted that this amount of energy depends on the physical conditions of the substance. In this class we will always be concerned with substances being measured under standard conditions of temperature and pressure [See Note]. The degree symbol (°) is often used to indicate that a measurement was made under standard conditions. Thus, you will often see the enthalpy symbol with a degree symbol, H°, to indicate that enthalpy was measured under standard conditions.
E N T H A L P Y
"heat content" of a systemSymbol for enthalpy: H
Unit for enthalpy:
the joule, JFor chemical reactions
it is more practical
to record enthalpy in kJ.We also refer to the change in enthalpy as the Heat of Reaction. The energy term you see as part of a chemical reaction is this "heat of reaction", or enthalpy change.
Heat of Reaction
ΔH
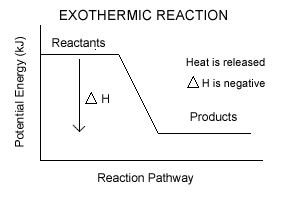
Exothermic Reactions
During exothermic reactions, more energy is released during bond formation than is required to break bonds. A typically exothermic reaction would be written like this:Cu(s) + Cl2(g) → CuCl2(g) + 220.1 kJIn this reaction, there is a net release of 220.1 kJ of energy, and the energy term (220.1 kJ) appears on the product side of the equation.Exothermic reactions release energy because the heat content of the reactants is greater than that of the products. We will think of this difference in terms of potential energy - in exothermic reactions, the reactants have more potential energy than the products. The "excess" energy is released to the surroundings during the reaction.
We can show this graphically as well. These graphs will be very important to you, so be sure you understand them well. The graph illustrates that the amount of potential energy stored in the products is less than that stored in the reactants; potential energy decreased during the reaction.
Think of this graph as a boulder rolling downhill - as it does, potential energy (the energy stored while the boulder is on top of the hill) is converted to kinetic energy (the rolling rock) which can do work (roll over and crush a house at the bottom of the hill).A potential energy graph of
an exothermic reaction:
We typically indicate that something has decreased by using a negative sign. For example, if the temperature drops 10°C, we would indicate that as a change of -10°C. An increase of 5°C would be recorded as a change of +5°C.
We do the same with enthalpy changes. When the amount of entropy in a system decreases a negative sign to indicate the drop. Thus, we could rewrite the equation shown above as:
With our new terminology, we say that the heat of this reaction was -220.1 kJ. During the formation of 1 mole of CuCl2(g) from 1 mole of Cu(s) and 1 mole of Cl2(g), 220.1 kJ of energy was released (given off to the surroundings).
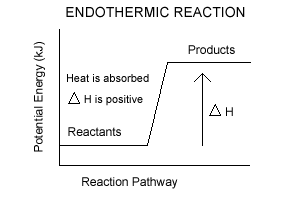
Endothermic Reactions
During endothermic reactions, energy is absorbed from the surroundings. A typical endothermic reaction is written with the energy term on the reactant side of the equation:H2O(g) + C(s) + 132 kJ → CO2(g) + 2H2(g)
A net input of energy is required to make this reaction occur.The potential energy of the reactants is lower than the potential energy of the products; energy must be supplied to the reaction in order for it to occur.
Think of this as the boulder being pushed up the hill. It takes energy to roll a boulder up a hill.
A potential energy graph of
an endothermic reaction
As with the exothermic reaction, we can remove the energy term from the equation and record the enthalpy change with a positive value, indicating that enthalpy increased during the reaction: