2.6 Bond Enthalpies
There is another way to calculate the heat of reaction, using bond enthalpies. Bond enthalpy refers to the amount of energy stored in the chemical bonds between any two atoms in a molecule. Bond enthalpies have been experimentally determined and can be found in a Table of Bond Enthalpies. |
|
The net change in energy during a chemical reaction is the difference between how much energy it takes to break chemical bonds and how much energy is released when bonds form. So if we know how much energy is stored in these chemical bonds, we can calculate the change in energy for a reaction. The energy change will equal:
Amount of energy required to break the bonds of the reactant molecules |
— |
Amount of energy released when the bonds of the products form |
If we know how much energy is stored in the chemical bonds, we can calculate the energy change of a reaction. It is important to realize that bonds are broken on the reactant side of the equation, and bonds are formed on the product side. This will give us an equation to use that is very similar to our Hess's Law formula, but with one important different - when using Hess's Law, we subtract the reactants from the products. But when using bond enthalpies, we will subtract the products from the reactants. Be careful to remember these differences.
Let's look at a few examples.
Given the following bond enthalpies:
| H — H | 436 kJ |
| Cl — Cl | 243 kJ |
| H — Cl | 433 kJ |
(you can find a more complete list of bond enthalpies in the bond enthalpies table)
Solution:
Draw structural formulas for all molecules. Pretty simple for these molecules:
Chemical formula |
Structural Formula |
H2 |
H — H |
Cl2 |
Cl — Cl |
HCl |
H — Cl |
Using the balancing coefficients in the balanced equation and the structural formulas, determine how much energy is required to break all of the bonds of the reactants, and how much energy is released when all of the product bonds form:
REACTANTS: BOND BREAKING |
PRODUCTS: BOND FORMATION |
||||||||||
Bond |
No. |
|
|
|
Total |
|
No. |
|
Bond |
|
Total |
|
|||||||||||
H - H |
1 |
× |
436 |
= |
436 |
|
|
|
|
||
Cl - Cl |
1 |
× |
243 |
= |
243 |
|
|
|
|
||
H-Cl |
|
|
|
1 |
× |
433 |
= |
433 |
|||
We still need to use the balancing coefficients from the balanced equation:
ΔH = Σ (bonds broken) – Σ (bonds formed) ΔH = [½(436) + ½(243)] – [(433)]
C2H4(g) + H2(g) → C2H6(g)
Given the following table of bond enthalpies:
| C — H | 413 kJ |
| C = C | 614 kJ |
| C —C | 348 kJ |
| H — H | 436 kJ |
Solution: Begin by drawing the structural formulas for all molecules.
Chemical formula |
Structural Formula |
C2H4 |
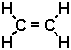 |
C2H6 |
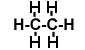 |
H2 |
H — H |
Determine how many bonds of each type are present in each molecule, and calculate the bond energies. For example, in C2H4 there is a single C=C bond (a double bond) but there are four C - H bonds.
REACTANTS: BOND BREAKING |
PRODUCTS: BOND FORMATION |
||||||||||
Bond |
bond enthalpy |
Number bonds per molecule |
Total Energy |
bond enthalpy |
Number bonds per molecule |
Energy |
|||||
C - H |
413 |
× | 4 |
= | 1652 |
413 |
× | 6 |
2478 |
||
C = C |
614 |
× | 1 |
= | 614 |
||||||
H - H |
436 |
× | 1 |
= | 436 |
||||||
C - C |
348 |
× | 1 |
= | 348 |
||||||
Finally calculate ΔH for the reaction, being sure to check for coefficients from the balanced equation:
C2H4(g) + H2(g) → C2H6(g) ΔH = Σ(reactant bonds) – Σ(product bonds)
ΔH = 2702 – 2826 = -124 kJ answer
|
Complete the assignment. |
