| 1. | Phosgene, COCl2, one of the poison gases used during World War I, is formed from chlorine and carbon monoxide. The mechanism is thought to proceed by:
a. Write the overall reaction equation. b. Identify any reaction intermediates. c. Identify any catalysts. |
||||||||||||||||||
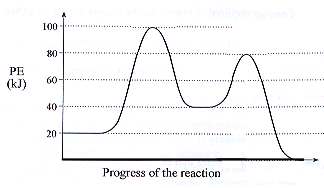
| 2. | We have typically been simplifying our potential energy curves somewhat; for multistep reactions, potential energy curves are more accurately shown with multiple peaks. Each peak represents the activated complex for an individual step. Consider the PE curve for a two-step reaction:
|
 |
|||||||||||||||||
|
|||||||||||||||||||
Chemistry 30
Thermodynamics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Kinetics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Equilibrium: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Solutions: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Acids & Bases: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Redox Reactions: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources