3.3 Changes in Volume & Pressure
Changing the pressure or volume of a container enclosing an equilibrium system will only affect the reaction if gases are present.
You may remember from earlier chemistry classes that equal volumes of gases contain an equal number of particles and, under standard conditions of temperature and pressure (STP), one mole of gas occupies a volume of 22.4 L. This is known as the molar volume of gases. So, two moles of any gas will occupy a volume of 44.8 L and one-half mole would occupy 11.2 L.
How does changing pressure and volume affect equilibrium systems?
- If you increase the pressure of a system at equilibrium (typically by reducing the volume of the container), the stress will best be reduced by reaction favouring the side with the fewest moles of gas, since fewer moles will occupy the smallest volume.
- Conversely, if you decrease the pressure (by increasing the volume of the container), equilibrium will shift to favour the side with the most moles of gas, since more moles will occupy a greater volume.
- If both sides of the equation have the same number of moles of gas, then there will be no change in the position of equilibrium.
When considering the effect of changing volume or pressure on equilibrium systems, be sure to only count the number of moles of GASES on each side of the equation. Solids, liquids, and aqueous solutions will not be affected by changing pressure and volume.
Here's an example.
Predict the effect on equilibrium when the pressure is increased for the following reaction:
N2O4 (g ) 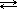 2 NO2 (g)
2 NO2 (g)
The reactant side of the equation has 1 mole of a gas; the product side has 2 moles. Increasing the pressure favours the side with the fewest moles of gas, so the equilibrium will shift to the left (the reverse reaction will be favoured).
