3.4 Changes in Temperature
When temperature is the stress that affects a system at equilibrium, there are two important consequences:
- an increase in temperature will favour that reaction direction that absorbs heat (i.e. the endothermic reaction)
- the value of Keq will change
Consider the following equilibrium system
N2O4 (g ) 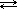 2 NO2 (g) ΔH° = +58.0 kJ
2 NO2 (g) ΔH° = +58.0 kJ
We see by the sign of ΔH° that the forward reaction is endothermic. Heat is absorbed (required as a reactant) when the reaction proceeds as
N2O4 (g ) → 2 NO2 (g)
By adding more heat, equilibrium will shift to use up the additional heat, thus favouring this forward direction.
Why will Keq change, when it did not change when concentration, pressure, and volume were the applied stresses?
When temperature changes cause an equilibrium to shift, one entire side of the reaction equation is favoured over the other side. Mathematically, this will alter the value of Keq as follows:
Keq |
= |
[products]
[reactants] |
|
if the forward reaction is favoured
|
more products are produced; fewer reactants
|
Keq will increase
|
if the reverse reaction is favoured
|
fewer products; more reactants
|
Keq will decrease
|
|
So in our example given above, increasing the temperature will favour the forward direction. The value of Keq will increase.
Removing heat (making the system colder) will favour the exothermic reaction - the exothermic reaction releases heat to the surroundings, thus "replacing" the heat that has been removed.