1.2 General Guidelines Concerning Solubility
If you add oil to water, what happens? Not much - the two don't mix,
even if vigorously shaken. Why can you clean some paints such as latex
paint and water colours with water, but oil based paints require a much
different type of cleaning solvent? Why do some substances readily form
solutions but others don't?
There is a general saying that "like dissolves like" (but this is a very general rule, with many exceptions). This phrase refers to the type of bonding between separate molecules, a topic you may have covered in earlier chemistry classes. If the bonding between separate molecules of the substance to be dissolved is similar to the type of bonding between solvent molecules, there is a good chance that the substance will dissolve. Time for a quick review of chemical bonding. We will examine both intramolecular and intermolecular bonding.
Intramolecular Bonding
Intramolecular bonding refers to the chemical bonding that holds atoms together within a molecule of a compound (see note 1). Covalent bonding and ionic bonding are the two main types of intramolecular bonding.
Covalent bonding involves the sharing of valence electrons between two atoms. For example, covalent bonding holds hydrogen and oxygen atoms together to form a water molecule, H2O. The sharing of electrons between atoms, however, is not always equal - sometimes the shared electrons sit closer to one atom than to the other, an important thing to know (see polar bonding below) .
Ionic bonding involves the transfer of valence electrons from one atom to another. One atom loses valence electrons, forming a positive cation, while the other atom gains electrons to form a negative anion. The electrostatic attraction between opposite charged ions holds the molecule (formula unit) together. An example is sodium chloride, NaCl, which involves the attraction between Na+ and Cl- ions.
Intermolecular Bonding
Intermolecular bonding, on the other hand, is what holds two or more separate molecules together in the solid and liquid phases. What type of intermolecular bonding is involved largely depends on two main factors:
- whether the bonds within a single molecular are polar or not (an unequal distribution of charge between two atoms involved in a chemical bond due to an unequal sharing of electrons), and
- the overall shape of the molecule (it's molecular architecture - tetrahedral, linear, bent, etc.).
A polar molecule will have one end of the molecule bearing a partial positive charge while another end carries a partial negative charge. Polar molecules must contain polar bonds.
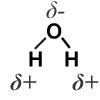
Water is an example of a highly polar molecule. Not only are the individual H—O bonds very polar (the shared electrons sit much closer to oxygen than to hydrogen, because oxygen has a higher electronegativity), but because the molecule has a bent shape the molecule itself is also polar. The oxygen end of the molecule has a partial negative charge (δ-), while the hydrogen end is partially positive (δ+).
What holds one water molecule tightly to the next is the strong attraction between the δ+ hydrogen end of one water molecule and the δ- end of a different molecule. |
 |
Nonpolar molecules either have no positive and negative ends, because the bonds making up the molecule are nonpolar, or because the entire outer "edge" is negative while the core of the molecule is positive (or vice versa), thus having no oppositely charged ends.
Forces holding two or more individual molecules together can be very weak (such as van der Waals forces) or involve strong dipole-dipole forces (an attraction between the positive end of one molecule and the negative end of another molecule). A special, very strong type of dipole-dipole force is hydrogen bonding. The attraction between two water molecules involves hydrogen bonding. This strong attraction between water molecules is what causes water to have a high surface tension.
Want more information about chemical bonding? Google search "chemical bonding".
Like Dissolves Like
Often, nonpolar substances dissolve best in nonpolar solvents (note 2), while polar substances dissolve best in polar solvents. The simple explanation is that the bonds between a solute particle and solvent molecule substitute for the bonds between the solute particles themselves.
Let's examine what happens when an ionic substance, NaCl dissolves in water. For our discussion we can think of ionic bonding as an extreme case of polar covalent bonding.
| In the solid form, the positive Na+ ions are strongly attracted to the negative Cl- ions. When added to water, the Na+ and Cl- ions become surrounded by water molecules, a process called hydration. The negative, oxygen end of the water molecule is strongly attracted to the positive Na+ ions, while the positive hydrogen end of the water molecule is strongly attracted to the negative Cl- ions. To keep things simple, you can imagine that the attraction of these ions to water is stronger than the bonds within NaCl itself, causing the Na+—Cl- to break apart. |
|
Not everything that dissolves in water, of course, forms ions. Sugar, a nonpolar substance, dissolves in polar water by forming hydrogen bonds with the water molecules. Ions, however, do not form.
Knowledge of the type of chemical bonding within a molecule can be useful in predicting whether a solute will dissolve in a given solvent, but because many exceptions exist, chemists must usually experiment to determine if two substances are indeed soluble with one another.
