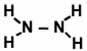| 1. | When iron rusts in air, the following reaction occurs: 4 Fe (s) + 3 O2 (g) → 2 Fe2O3 (s) ΔH = -1643 kJ What is the heat of formation, ΔHf, of Fe2O3? (hint: what is the heat of formation reaction for Fe2O3? How does this reaction compare to the reaction given in the question?) |
||||
| 2. | The equation for the decomposition of mercury(II) oxide is as follows: 2 HgO (s) + 182 kJ → 2 Hg (l) + O2 (g) Determine ΔHf for HgO (s) ? (hint: What does ΔHf mean? What would be the overall equation associated with ΔHf ?) |
||||
| 3. | Using a table of thermochemical data, calculate ΔH ° for the following reactions. Show your work. | ||||
| a. | CO (g) + ½ O2 (g) → CO2 (g) | ||||
| b. | CH4 (g) + 2 O2 (g) → CO2 (g) + 2 H2O (g) | ||||
| c. | CS2 (g)+ 2 H2O (l) → CO2 (g) + 2 H2S (g) | ||||
| 4. | Calculate ΔH ° for the process: 2 Al(s) + Fe2O3 (s) → Al2O3 (s) + 2 Fe (s) Given that ΔHf° of Fe2O3 = -813.0 kJ/mole and ΔHf° of Al2O3 is -1,655.0 kJ/mol |
||||
| 5. | The standard heats of formation for CH4, CHCl3 and HCl are -74.8, -132, -92.0 kJ/mole, respectively. Use this information to calculate the heat of reaction for the following reaction: CH4 + 3 Cl2 → CHCl3 + 3 HCl |
||||
| 6. | The standard heat of formation, ΔHf, for C2H4 is +52.3 kJ/mol. If C2H4 (ethylene) reacts with H2 to produce C2H6 (ethane) according to the following equation: C2H4 + H2 → C2H6 ΔH = -137 kJ what is the heat of formation of C2H6? (Hint : Solve this question without looking up ΔHf° for C2H6 in the Table of Thermochemical Data, although you may want to in order to check your answer. Solve this using the standard formula, ΔH reaction = ∑ ΔH products - ∑ ΔH reactants. However, this time you know ΔH reaction and need to solve for a substance on the product side of the equation.) |
||||
| 7. | Using bond enthalpies, calculate ΔH for the following reaction: N2 + 2 H2 → N2H4 The structural formulas for all reaction participants are shown here: |
||||
|
|||||
| 8. | The energy from the combustion of hydrazine, N2H4 , is used to power rockets into space in the reaction: N2H4 (g) + O2 (g) → N2 (g) + 2 H2O (l) ΔH ° = -627.6 kJ/mol How many kilograms of hydrazine would be necessary to produce 1.0 × 108 kJ of energy? Hint: One mole of N2H4 produces 627.6 kJ of energy. How many moles (and then grams) are required to produce 1.0 × 108 kJ of energy? |
||||
Chemistry 30
Thermodynamics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Kinetics: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Equilibrium: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Solutions: Unit Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Acids & Bases: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources
Redox Reactions: Module Index | Practice Problems | Assignments
| Student Lab | Research Ideas | Teacher Resources