4.2 Factors Influencing Reaction Rate: Temperature
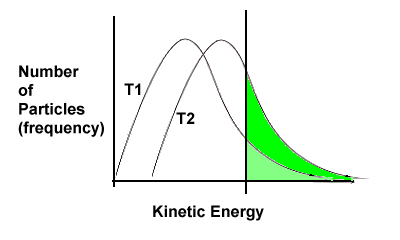
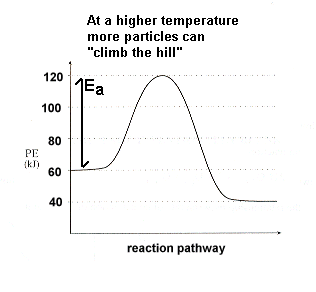
The rate of almost all chemical reactions increases if the temperature of the system is increased. A general rule of thumb is that the rate will double with an increase of 10°C (but "rules of thumb" are not iron-clad rule; exceptions exist).
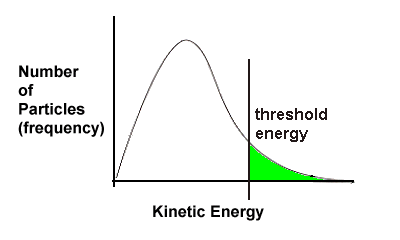
The collision theory helps us understand why this occurs. Recall the kinetic energy curves from Thermodynamics Section 1-3 and earlier this unit (Section 2-3):